Our non-profit association “Orphan Medicine” – Promoting Access to Care with Essential Medicine (PACEM) e. V.) has conducted clinical research for almost 20 years to find out how the cure of skin leishmaniasis in the so-called Old World can be made more efficient, i.e. cosmetically more acceptable and cheaper than the extremely painful chemotherapy with penta-valent antimony preparations practiced for over 50 years. The Leishmania parasites, which trigger the skin lesions, are transferred by bloodsucking sand flies to the human skin mainly in the face and again predominantly in small children with skin that is easy to pierce, where they multiply in the large defence cells, the skin macrophages, whose defence they can resist and which are thereby prevented from contributing to wound healing.
Two Phase II studies published in international peer-reviewed journals (PLOS and BMC) and cited by the National Institute for Health and Care Excellence (NICE) have shown that skin leishmaniasis in its ulcerous form is a chronic wound disease. It is by no means fatal, except in exceptional cases of immunodeficiency. Antimony chemotherapy does not completely eliminate the parasite from the human body, there is no sterile cure. Rather, remaining parasites survive in the cells of our reticulo-endothelial system including the macrophages, a cell system that is so important for our non-specific immune defence. Even if no wound treatment is carried out, at the end of months or years there is spontaneous healing, but with a more or less disfiguring, lifelong unaesthetic result. These scars stigmatize girls and exclude women from very conservative societies in countries such as Afghanistan, Pakistan and some Arab countries.
The recommendation of KITAB EÇ ÇIH’HA in the book of good health, “Le Livre de la Bonne Santé”, published in Algeria in 1922, was forgotten with the advent of antimony chemotherapy over 50 years ago for open lesions of skin leishmaniasis in the Old World. We have tried to comply with these recommendations, taking into account the hand hygiene recommended by the WHO. This method is able to heal complex lesions in parts of the body that are difficult to treat with topical antimony within a few weeks. In a more economical and less painful way, this leads to better aesthetic results and shorter healing times.
With our pilot results in the L. major Leishmania endemic area of Bou Saada and M’Sila in Algeria, we have applied for the special approval according to § 11 MPG (Medizinproduktegesetz, Legislation Governing Medical devices ), which only exists in EU countries such as Germany, for our LeiClean treatment. We have been granted this special approval which now been extended until September 2020 for the second time. This special approval is temporary and does not replace the CE certification by the TÜV (Technical Control Board) needed for the production of medical devices in Europe.
Even if the responsible manufacturer, as in our case, is a non-profit association, he is liable to pay the costs and such costs will turn around 60,000 €. The CE certification is necessary so that Leishmania endemic countries can produce the materials necessary for the LeiClean treatment in their own country for their Leishmania patients after they have reached a local validation of the CE certification.
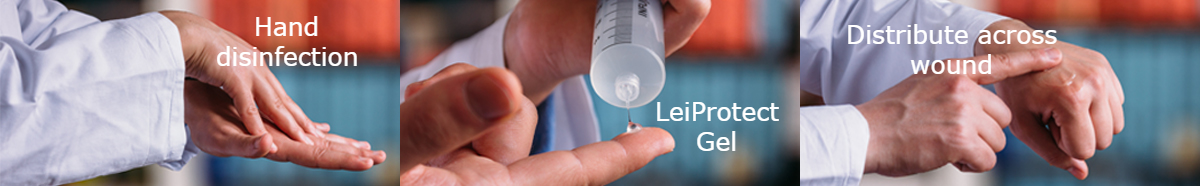
LeiClean means that users of the film-forming gel LeiProtect®, a class IIb medical device, first disinfect their hands with hydroalcoholic solution as recommended by the WHO before covering their skin wounds with the film-forming gel LeiProtect®. LeiProtect® was developed by the pharmacists and doctors of our Orphan Medicine Association in Afghanistan at the beginning of 2014.
The special approved gel LeiProtect® Gel is manufactured by the GMP-certified hospital pharmacy of the University of Erlangen as OEM manufacturer for the Orphan Medicine e. V. as responsible manufacturer. The costs of the small 10 x 1 kg batches of LeiProtect® produced with a YSTRAL jet streamer according to GMP are still relatively high. They currently amount to 1 € per 1 g gel, which currently leads to daily treatment costs of 0.1 to 0.2 € per skin lesion, which are expected to decrease considerably, i.e. by approx. 90%, in the production of 1,000 kg batches. The Conti-TDS Jetstreamer is a material donation with a new acquisition value of 70.000 € to the non-profit orphan medicine registered association.
Our preliminary results of a compassionate use observation in 35 patients with 45 complex skin leishmaniasis lesions from Algerian patients who had opted for LeiClean treatment because they did not want to undergo two weeks of hospital treatment with i. m. injections of pentavalent antimony, showed that an outpatient cure of Leishmania skin lesions could be achieved even without antimony chemotherapy. Half of the patients showed healing of their complex Leishmania lesions after 4 weeks, with an average of 7 € per lesion for the LeiProtect® costs at their actual production price. In comparison: The costs of a three-week hospital treatment with pentavalent antimony were investigated in a recent publication published in Morocco (Tachfouti N, Najdi A, Alonso S, Sicuri E, Laamrani El Idrissi A, Nejjari C, Picado A). Costs for the treatment of pediatric visceral leishmaniasis in Morocco. PLoS One. 2016 Jun 3;11(6):e0155482). Inpatient treatment costs were estimated at USD 510 per patient.